1 Which of the Following Compounds Is Not Aromatic
According to this rule aromatic compounds must satisfy the following points-. State the reason for your answer.

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps
Check Answer and Solution for above.

. Hence the correct option is option A. By Huckels rule 4n 2 4. CH-CH2 CH3 CH3-CH2 b.
It is not equal to 4n2. Answers 1 n - alkane having six or more then six carbon atoms on heating to 773 K at 10 to 20 atmospheric pressure in the presence of oxides of vanadium molybdenum or chromium supported over a lumina get dehydrogenated and cyclised to benzene and cyclised to benzene and its homologes. Hence compoundsions of options B and D are aromatic.
CH3 H CH3-CH2 H ос H CH2-CH3. The ion of option C has 2 pi. So they cannot be aromatic sea is already cancelled now.
B Not planar Hence it is non-aromatic. Okay so if you check in a option this is not following foreign plus two rules. And the option.
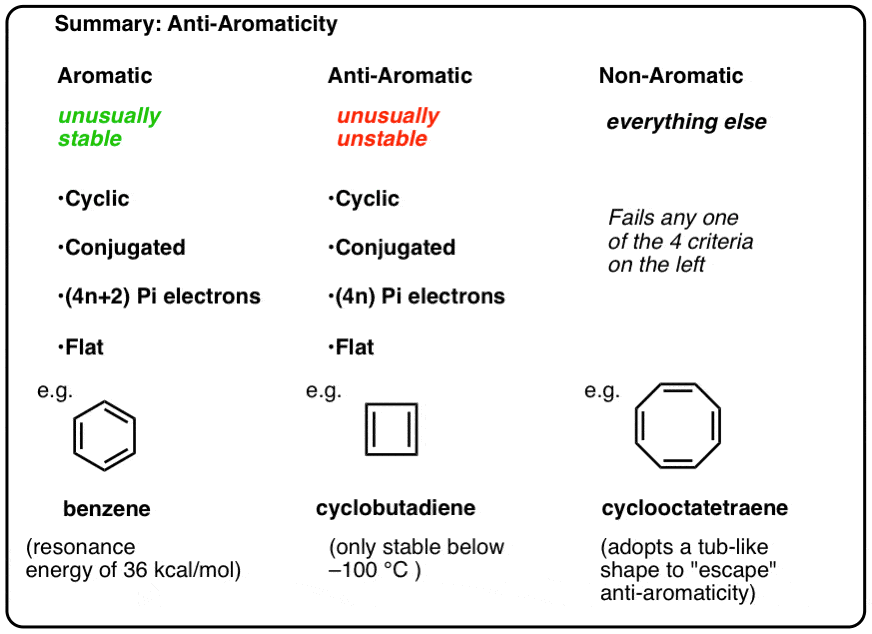
B is anti-aromatic with 4 π e. Anti-aromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised π or lone pair electrons in it. D is non aromatic because of sp 3 carbon in the ring.
Aromatic compounds have extra stability with comparing to other nonaromatic or aliphatic compound and the aromatic compounds obey Huckels rule. See we doesnt have to check and then be There is 1 2 and three. Identify the following compounds as aromatic or not aromatic.
Ii For the given compound the number of π-electrons is four. Which of the following compounds is not. Correct option is A According to Huckels rule the planar cyclic conjugated structures having 4n2π electrons where n is 012etc are considered as aromatic compounds.
Hence cycloheptatrienyl anion is not an aromatic compound. In cyclopentadienyl cation A resonance takes place. Option 4Has 6π electrons in conjugation but not in the ring.
Determine if the following compounds are AROMATIC ANTIAROMATIC or NOT AROMATIC Cyclobuta-13-diene Naphthalene Pentalene Acenaphthylene Phenanthrene Biphenylene Anthracene Chrysene Tetracene This problem has been solved. Aromatic structure is defined as those structure observed in organic compounds known as aromatic compound. It is cyclic not planar.
Unlike aromatic compounds which follow Hückels rule 4 n 2 π electrons and are highly stable anti. C is anti-aromatic with 8 π e. Asked Jan 5 in Chemistry by ShivrajSharma 249k points class-12.
Correct option is A a Number of π electrons 4. Provide missing reagents for the following reactions. Hence it is non aromatic in nature.
Hence the given compound is not aromatic in nature. Co-ordinations compound 366 Environmental chemistry 9 Purification and characteristics of organic compounds 1 Some basic principles of organic chemistry 12 Hydrocarbons 186 Organic compounds containing halogens 100 Organic compounds containing oxygen 140 Organic compounds containing nitrogen 126 Polymers 117. What is the decreasing order of reactivity amongst the following compounds towards aromatic electrophilic substitution I.
Which one of the following compounds is non-aromatic. Orbitals of one carbon atom are not in conjugation. The compoundsions in options B and D have 6 pi electrons.
Option 2Has 6π delocalized electrons. There are six electron pairs. CH3-CH-CHCH2 In the IUPAC name for the following compound the -Br group is located at position Br Select one.
I mainly need help with number 2 and thanks in advance. Which of the following compounds is not aromatic. Okay because this is following foreign rule and foreign rulers for anti aromatic not aromatic.
The correct option is 1 B C and D. Those compound whom do not have 4n2 It has electrons. It does not obey huckles rule.
For cyclopenta-13-diene anion G It is cyclic planar and has 6π conjugated electrons that means all the pi electrons are in continuous delocalization hence we consider negative charge in conjugation. They are cyclic planar and conjugated systems of pi electrons. What is the decreasing order of reactivity amongst the following compounds towards aromatic electrophilic substitution I.
It is not an aromatic compound. For a compound to be aromatic the value of n must be an integer n 0 1 2 which is not true for given compound. However cyclobutadiene have 4nπ electrons which are involved in cyclic delocalization are referred to as anti aromatic compounds.
It is not aromatic. In the last we can conclude that phenol naphthalene and pyridine are aromatic compounds. Option 3Out of 8π electrons it has delocalized 6π electrons in one six-membered planar ring which do not follows Huckels rule due to which it will be non-aromatic.
Which of the following compounds is not aromatic. Aromatic compounds are characterised by their unusual stability delocalisation of π -electrons in a planar ring. They undergo substitution reactions more favourably than addition reaction these properties are not present in cyclohexane.
For 78 dihydronaphthalein H It is cyclic planar but the electron cloud is not in. Hence it is aromatic. Benzenoids are the compounds having at least one benzene ring.
2 0 03 O d4 Which of the following compounds is trans-3-hexene. A Image A B Image B C Image C D Image D. Hence is not aromatic.
Asked Jan 5 in Chemistry by ShivrajSharma 249k points class-12. Which of the following compounds is not considered to be aromatic. Aromatic compounds are categorized further into two categories ie.
So the correct answer is Option D.

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps

Among The Above The Number Of Aromatic Compound S Is Are
Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry
No comments for "1 Which of the Following Compounds Is Not Aromatic"
Post a Comment